Jim Haseloff (Plant Sciences, University of Cambridge), Francesco Ciriello (Mathworks, Cambridge) and Maziyar Jalaal (Department of Applied Mathematics and Theoretical Physics, University of Cambridge).
OpenPlant Biomaker Design2020
Modern biology has exploited enzymes and clever reaction systems to produce an ever-growing range of diagnostics, environmental assays, DNA assembly and tools for education and research. Many of these molecular reactions are cheap and accessible, but require instrumentation for precise temperature regulation, programmed control over time and quantitative readout. Commercially available instruments are expensive, employing milled metal components for temperature control of hundreds of samples. There are many cases where low cost (<<£100) reactors would be very useful, even if sacrifices were made for the scale of sample numbers or flexibility or speed of temperature control.
We have taken on the challenge of exploring new open solutions for ultra-low cost micro-incubators. At the beginning of the COVID-19 lockdown, we set up an international Biomaker expert group to tackle this. Currently, around 75 participants and observers are connected via web exchange and regular teleconferencing. We have built: (i) a programmable rig for testing prototypes. The integration of no-code programming and programmable touchscreen means that the instrument can be highly flexible, and easily repurposed. (ii) This has inspired the assembly of a small control unit that can be used for low-cost implementation, after the testing process. (iii) Quantitative models for air flow and heat transfer to inform new designs and computer control. Air heating/transfer can potentially provide a cheaper and more accessible route to incubator design, compared to the conventional use of heaters attached to milled aluminium blocks. (iv) We have incorporated cheap heating hardware into custom 3D printed housings and are testing the designs.
Quantitative modelling
Figure 1: The control system for the simulated instrument (©MathWorks).
3D printing allows the design and low-cost testing of possible solutions that can include high levels of geometric complexity. One of the challenges with building 3D printed incubators is the prospect of softening or melting of thermoplastics - in our case, we'd ideally like to have a working range of zero to 95 degrees C - to encompass most commonly used DNA procedures. One of the expert group, Carlo Quinonez, has pioneered the use of 3D printing techniques to experiment with complex manifolds and flues for microscope-based cell culture incubators - and to ultimately build a high-temperature (190 degrees C) convection oven using heat resistant ULTEM 1010 plastic (https://studiofathom.com/blog/pyrahttps://www.instructables.com/id/Shape-of-things-to-come/), and we have taken inspiration from this approach.
LAMP, PCR and other incubator reaction protocols require precise and often dynamic temperature control. The thermal response of a reaction vessel, and as a result the performance of an incubator, depends on a variety of factors. These include:
• the material properties of the device components, e.g. their thermal conductivity, density and specific heat capacity;
• and of the fluid surrounding them, e.g. its thermal diffusivity, viscosity, density;
• the geometry of these components, e.g. their surface areas, volumes, thicknesses, shapes, arrangements;
• the ensuing flow patterns that result from the convection of heat along their surfaces, e.g. typified by spatially-varying flow velocities, temperature gradients and flow regimes (laminar vs turbulent flows, forced vs natural convection).
The complex nature of convective flow patterns presents many challenges to the successful design of an incubator. The correct estimation of heat transfer coefficients for components often requires detailed experimental or numerical studies. Fortunately, there is a wealth of literature when it comes to the documentation of these studies (Holman 2009) and results for simplified geometries can be drawn upon to obtain sensible estimates for these coefficients. These idealisations can inform design choices at an early stage. Relying on these, a modelling framework can be established that can successively be improved, moving forward, by a combination of hardware testing and supporting high-fidelity FEA and CFD simulations.
To build a theoretical foundation for the design, Francesco Ciriello drafted a simplified thermal model of an incubator in Simscape. Simscape is a modelling tool within the Mathworks Simulink product family that allows for the rapid design of 1D lumped-mass models for multi-domain systems.
Figure 2: Model variants were used for the controller and plants to systematically assess the impact of specific design choices on the thermal response of the samples (©MathWorks).
Lumped thermal masses are used to idealise the samples, the air in the chamber and the reaction chamber walls. Heat transfer coefficients and resistances are extracted from Holman’s book (Heat Transfer. 10th Edition, McGraw-Hill, New York, 2009) Heat Transfer book for different geometries and scenarios. The simulated instrument can now be used to experiment with design choices and controller design and tuning (Figure 1). Simulink model variants have been particularly helpful at enabling these comparisons (Figure 2).
The simulations are encouraging us to explore different parameter spaces and question traditional design paradigms, e.g. the use of resistance heaters strapped to milled aluminium blocks. Simulations are also empowering when access to hardware resources is limited. The combination of Model-Based Design, rapid prototyping with additive manufacturing technologies and low-cost electronics are a powerful enabler for collaboration on open-science projects and can democratise access to a wealth of laboratory resources for biological research and medical diagnostics.
Mazi Jalaal has used Computational Fluid Dynamics to explore important parameters, such as heat transfer coefficients, in the model explained above. He used the CDF package Comsol to simulate the fluid flow and heat transfer inside an incubator. Figure 3 shows an example of the simulations, where warm air entering the closed incubator heats a series of reaction vessels.
Figure 3: Heating eight reaction vessels inside an incubator. Colours show the temperature field
Mazi Jalaal has used Computational Fluid Dynamics to explore important parameters, such as heat transfer coefficients, in the model explained above. He used the CDF package Comsol to simulate the fluid flow and heat transfer inside an incubator. Figure 3 shows an example of the simulations, where warm air entering the closed incubator heats a series of reaction vessels.
Figure 4. Test-rig and compact control unit
Test-rig and compact control unit
One challenge was to build a test-rig that would combine the flexibility of microcontroller-based electronics with code-free programming in XOD - and speed up the development and testing of control routines and graphical user interfaces. Jim Haseloff has built a testing device with integrated programmable touchscreen to simplify design and prototyping of the instrumentation. The screen manufacturers provide software that allows drag-and-drop programming of multi-screen user interfaces, with a corresponding set of Arduino libraries. Further, XOD programmers have converted the Arduino IDE libraries to XOD libraries, with a set of nodes that allow simple visual coding of interactions between the microcontroller and screen. For example, the touchscreen can provide multiple sets of switches, knobs, gauges and level settings and sophisticated display of parameter settings or sensor measurements - all quickly reconfigurable in software. The test-rig provides a base for controlling and monitoring prototype instruments. In order to then adopt the interface for real-world instrumentation, we're experimenting with compact lower-cost hardware that can be used to build standalone instruments.
Figure 5. Components chosen for prototype, with 200µL 8-tube strip for reference.
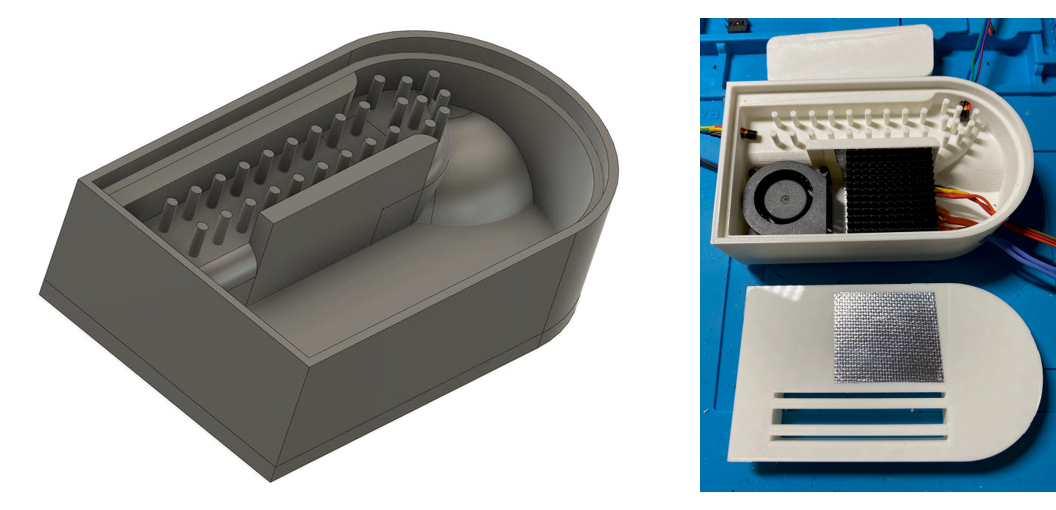
Figure 6. 3D design for reactor vessel (left) and printed prototype with hardware installed (right).
Components for a simple prototype microreactor
Jim Haseloff selected a simple set of low-cost hardware components: PTC heater, 40mm fans, 40mm heatsinks, MOSFET modules for computer controlled switching, DS18B20 pre-calibrated digital temperature sensors and 12V DC power supply (total ~$25). These were assembled in 3D printed housings that had been designed in Autodesk Fusion 360, printed on an Ultimaker S3 printer, and were tested using the programmable test-rig assembled for this purpose.
The prototype incubator was designed for heating small (0.2 µL) reaction tubes to around 60o Celsius for LAMP diagnostics. To minimise costs and complexity, a positive temperature coefficient (PTC) heater was used as a heat source. PTC heaters provide resistive heating. When cool, the device draw a relatively large current. As the temperature rises, the resistance increases - resulting in a fall in current. This continues until the heater reaches a maximum temperature. Therefore PTC heaters do not overheat, and this simplifies the design of both the prototype control system and vessel. The prototype was intended to exploit air heating (rather than metal block heating) and vessel shape and baffles to maximise efficient heat exchange with the incubator.
Figure 7. Real-time monitoring through digital temperature sensors. Upper right panel shows the plotted output of DS18B20 temperature sensors in a prototype incubator, showing heating from room temperature (24 degrees C) to a target of 60 degrees. The heater output was controlled from XOD, and the temperature increase was logged onscreen. (The x-axis graticule marks are 20 secs apart).
Figure 8. Visualisation of heat transfer through IR sensing.
The performance of the prototype was monitored in realtime through digital temperature sensors and infrared emissions. Real time plots of DS18B20 temperature sensors in a prototype incubator show heating from room temperature to a set point of 60oC in two minutes. The microreactor could accurately maintain temperatures to within a degree at set points from 37o to 60o Celsius. This has been an encouraging start to the project, given the cost of the components, and with many avenues still to explore for improvement for efficiency, speed, cycling and real-time measurement of reactions in situ. A manufactured microreactor of this type would be cheap and find wide use for diagnostics, education and science, especially in low resource settings.
Further information: Test-rig assembly - https://www.hackster.io/jim-haseloff/programmable-test-rig-d7df62 Prototype microreactor - https://www.hackster.io/jim-haseloff/airloop-i-5d2a72 Biomaker - https://www.biomaker.org Biomaker Expert Group - contact Jim Haseloff at jh295@cam.ac.uk if you are interested in getting involved. All welcome!